CS Analytical operates in the highly regulated Pharmaceutical, Biotechnology and Medical Device marketplaces. As such, the facility and operations must adhere to a strict quality system that ensures that programs and processes meet guidelines as depicted by ALCO+ Principles
Attributable
Contemporaneous
Accurate
Consistent
Available
Legible
Original
Complete
Enduring
ALCOA+ is a set of principles that ensures data integrity in the life sciences marketplace, and it is a key component that is reviewed and enforced by the FDA (Food & Drug Administration). These principles are applied to a range of areas, particularly in relation to pharmaceutical research, manufacturing and testing. They play a key role in helping to ensure GMP compliance.
To help ensure that the CS Analytical Team meets these critical regulatory required expectations, a number of key quality credentials and certifications are maintained.
CS Analytical is an FDA registered analytical testing laboratory: FEI # 3017927136. Work performed for submission to the FDA is done in compliance with cGMP guidelines (21 CFR Parts 210 & 211) as applicable to a contract analytical testing laboratory. Key components of FDA registration include:
GOOD MANUFACTURING PRACTICES
CA Analytical has implemented current Good Manufacturing Practices (cGMPs) as stated in the code of Federal Regulations. These regulations apply to the facility or controls to be used for the “Manufacture, Processing, Packing or Holding of Drugs;” Although our facility does not perform these functions and are therefore not subject to these practices, we do operate within the following sections of the regulations: 21 CFR Subpart 11; 21 CFR Subpart 210; 21 CFR Subpart 211; 21 CFR Subpart 58; 21 CFR Subpart 820.
DEBARMENT CERTIFICATION
CS Analytical has not been debarred by the FDA nor is currently involved in any debarment proceeding with the FDA. Determined by a signed and dated certification statement, no person employed by the laboratory has currently or in the past five years been convicted of any crime described in Sections 306 (a) or (b) of the Generic Drug Enforcement Act of 1992. It is written per our internal quality system that CS Analytical has not, and will not, use the services of any person debarred under Section 306 of the Generic Drug Enforcement Act of 1992.
In additions, CS Analytical also holds the following certifications:
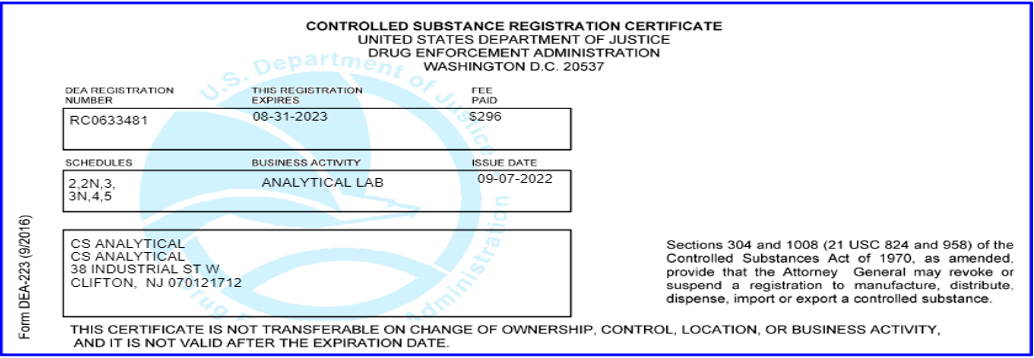
Controlled Dangerous Substances Schedules 2 through 5:
International Safe Transit Certified Testing Laboratory

The FDA registration along with these key certifications help to ensure that all work performed by the CS Analytical Teams meets or exceeds all regulatory requirements for the clients we serve. Our commitment to quality never wavers regardless of the job at hand. It is this approach and culture that helps to ensure that each test request completed meets the robust requirements that ALCOA+ dictates.

