Magna Mike Thickness Guage Testing
By Meaghan Mancini / Laboratory Analyst II The Magna
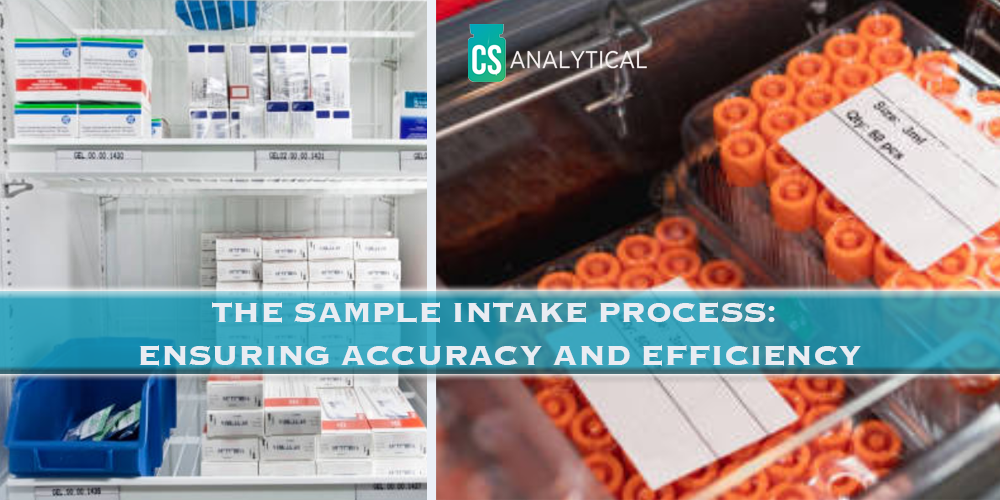
The Sample Intake Process: Ensuring Accuracy and Efficiency
Written By Jacqueline Zsoldos / Quality Administrator When samples
Understanding Cleaning Validation in Pharmaceutical Manufacturing
Written By Ronak Patel / Manager, Analytical Chemistry Cleaning
A history of the United States Pharmacopeia (USP)
Written By Rohan Kumar / Laboratory Analyst 1 Author
Ensure Your Plastic Materials of Construction and Package Systems are Qualified Under USP 661.1 and 661.2 Without Delay.
Written By Ronak Patel, Manager, Analytical Chemistry The clock
Understanding USP 660 Glass Testing – Autoclave Use and Test Timing
Written By FATIMA YACOUBI, Laboratory Analyst III / Project
USP TOC Testing for Plastic Container Systems
Total Organic Carbon (TOC) testing is an established USP test
cGMPs and Their Importance in Contract Laboratories
The life science industry is built upon a foundation of
ASTM 1980 Accelerated Aging Testing
The ASTM F1980 Standard Guide for Accelerated Aging of