 The ASTM F1980 Standard Guide for Accelerated Aging of Sterile Medical Device Packages is a test procedure that can be useful in the qualification of a wide range of container and package systems. While the procedure title is specific to Sterile Medical Package Systems, this test method is routinely used on a host of package systems regardless of their sterility requirements. As clearly stated in the ASTM F1980 procedure, “Accelerated aging studies provide an alternative means” in place of real time aging studies that help get products to market in the shortest time possible. In addition, the application of accelerated aging testing is referenced in a number of other compendial procedures and regulatory guidance documents: ANSI/AAMI/JSO II 607 states that, “the manufacturer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package is maintained at least for the claimed shelf-life of the medical device under storage conditions specified by the manufacturer, as long as the package is undamaged or unopened.” In this article we will outline some of the key components required to run an effective accelerated aging study.
The ASTM F1980 Standard Guide for Accelerated Aging of Sterile Medical Device Packages is a test procedure that can be useful in the qualification of a wide range of container and package systems. While the procedure title is specific to Sterile Medical Package Systems, this test method is routinely used on a host of package systems regardless of their sterility requirements. As clearly stated in the ASTM F1980 procedure, “Accelerated aging studies provide an alternative means” in place of real time aging studies that help get products to market in the shortest time possible. In addition, the application of accelerated aging testing is referenced in a number of other compendial procedures and regulatory guidance documents: ANSI/AAMI/JSO II 607 states that, “the manufacturer shall demonstrate that, under the rigors of distribution, storage, handling, and aging, the integrity of the final package is maintained at least for the claimed shelf-life of the medical device under storage conditions specified by the manufacturer, as long as the package is undamaged or unopened.” In this article we will outline some of the key components required to run an effective accelerated aging study.
Key Test Concepts to Understand: Accelerated aging of materials refers to the accelerated variation of their properties over time, the properties of interest being those related to safety and function of the material or package. In an aging study, the material or package is subjected to an external stress, which is more severe, or more frequently applied than the normal environmental stress, for a relatively shorter period of time. Accelerated aging techniques are based on the assumption that the chemical reactions involved in the deterioration of materials follow the Arrhenius reaction rate function. This function states that a 10°C increase or decrease in temperature of a homogeneous process results in approximately, a two times or ½-time change in the rate of a chemical reaction. Key test factors are TIME and TEMPERATURE.
Key Definitions:
Accelerated Aging – the storage of samples at an elevated temperature designed to simulate real time aging in a reduced amount of time.
Accelerated Aging Factor – estimated or calculated ratio of the time to achieve the same level of physical property change as a package stored at real time conditions.
Accelerated Aging Temperature – the elevated temperature at which the aging study is conducted, and it may be based on the estimated storage temperature, estimated usage temperature, or both.
Accelerated Aging Time – the length of time the accelerated aging study is conducted for.
Accelerated Aging Test Preparation Steps:
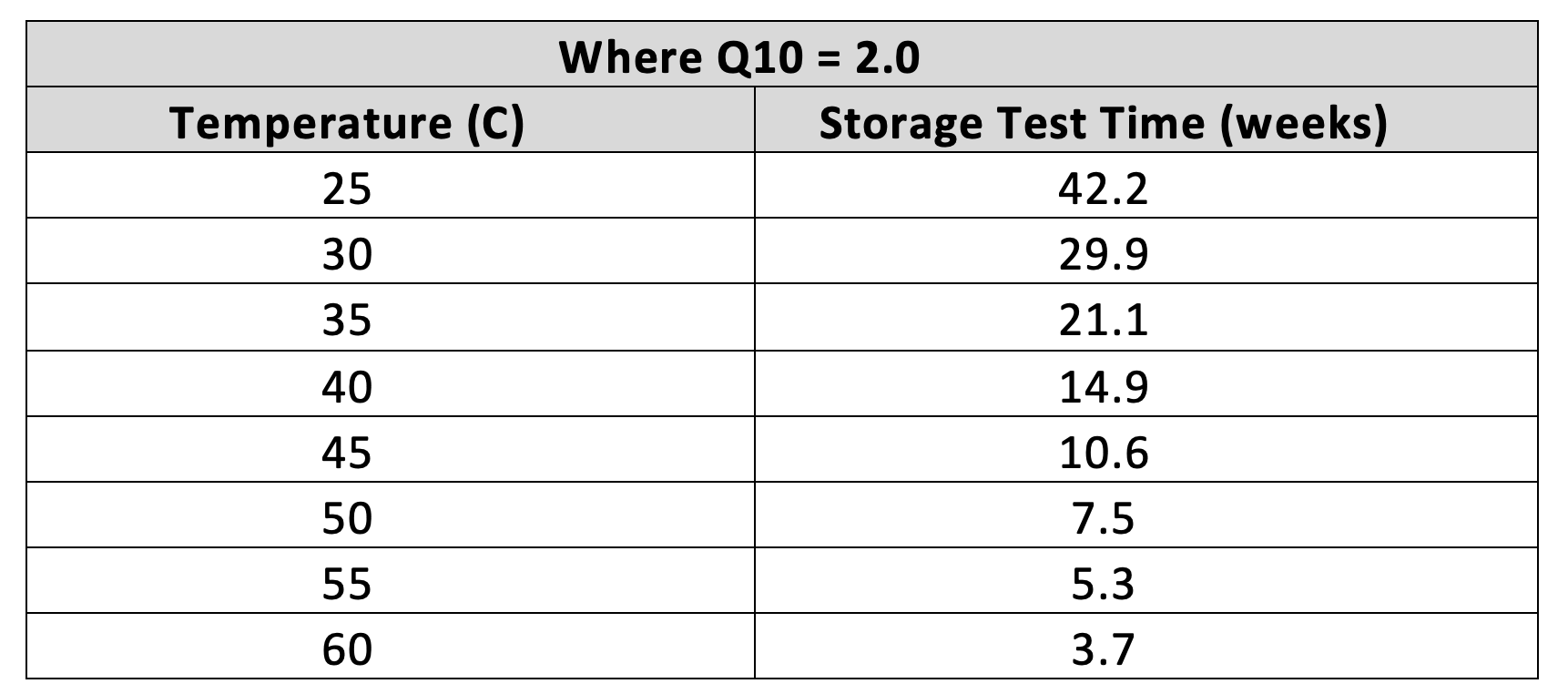
While a read through of the ASTM F1980 test procedure may seem a bit convoluted and complicated as they outline a number of unique equations for determination of critical parameters, the actual set-up and performance of the testing is relatively simple, The key factor is determining the temperature and associated time period for storage at an elevated temperature that the samples will be subjected to. The first critical steps is knowing the material of composition of the samples and the intended normal storage condition they will be endure during market use. When selecting the temperature to be used, the higher the temperature the shorter the accelerated aging storage time. However, it must be noted that a temperature that could have an abnormal effect on the sample should not be used. This is especially true of polymeric materials where elevated temperatures may have an effect on the samples that may never be seen during real time or room temperature use. As directed in the ASTM procedure, a temperature in excess of 60o C should never be used. Once you determine the temperature set point, based upon the Q10 factor being equal to 2.0, you can determine the storage time period. The easiest way to determined this is referencing the table and chart depicted in the ASTM F1980 procedure:
Once the storage parameters have been established, prior to storage and testing, the associated sample evaluation method(s) must be defined and the acceptance criteria must be set. In many cases, visual inspection may serve as the evaluation test method. However, other test methods may be utilized to test specific parameters of the package system performance. For example, ASTM F88 Seal Strength Testing may be used to evaluate changes in seal integrity. In other instances, ASTM Bubble Leak or ASTM Burst & Creep testing may be used. Regardless of the test method to be used, the acceptance criteria must be established prior to performing the actual accelerated aging study. In some instances, pre-storage testing to establish acceptance criteria may be performed.
Once the program is completed a formal report should be issued that outlines all relevant test steps to include the instrumentation used (inclusive of calibration and performance verification data), the specific test methods used for sample evaluation and results for each sample specific to the pre-defined acceptance criteria.
The CS Analytical Team has many years of experience defining and executing accelerated aging studies on a wide variety of container and package systems. Their knowledge and experience can help you define a program that meets your product needs and aligns with all established regulatory guidelines.

