Assembling the Package Integrity Profile for Autoinjectors
Due to their complexity, combination products, specifically autoinjection systems, present unique challenges in implementing a container closure integrity (CCI) testing strategy with reference to USP <1207>. This is further complicated by a pervasive, though diminishing, tendency for companies to consider CCI only at the final product-package lifecycle stage, an approach where combination products will highlight the pitfalls. This article seeks to discuss the unique challenges of CCIT on combination products, and how to overcome them using a modern, risk management approach to CCI in line with current guidance inclusive of USP <1207> and regulation: development of a package integrity profile.
Prefilled Syringes and Autoinjection Systems
Over the past two decades, there have been substantial changes in the way that container closure integrity is viewed and implemented for product-package systems, exemplified by a fully revised USP Chapter <1207> released in USP 39 in 2016 after years in public forum and debate. In those same few decades, there has been substantial growth of more complex dosage forms and use cases, in turn expanding packaging and systems beyond the traditional glass vial and rubber stopper, namely the prefilled syringe.
The pre-filled syringe has numerous benefits. From a technical and business perspective, PFS systems reduce potential for product waste, and may reduce costs in some cases, such as those previously requiring a vial kit. From and end-use standpoint, they have a pre-measured dose, reducing the risk of error, and can be administered in a professional setting or self-administered by the patient in the course of their daily life. In fact, PFS has been and continues to be the largest growth sector in the pharmaceutical primary package space, with projected growth from around 4.8 billion in 2018 to upward of 9 billion by 20251, though exact numbers vary according to market research firm.
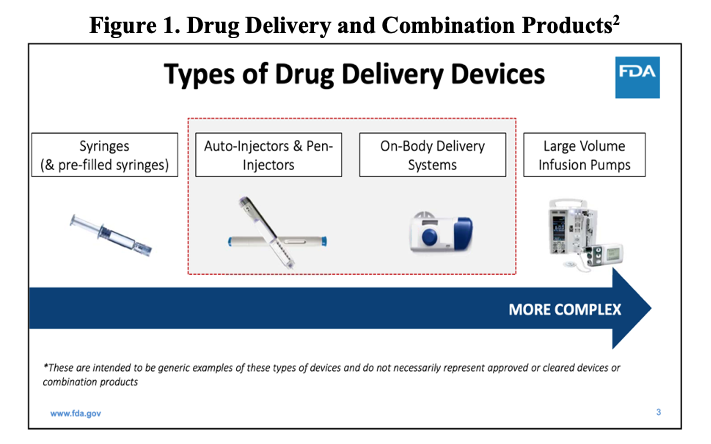
While the PFS is considered a primary package, as it is containing the drug, it is also considered a combination product: the syringe is a device being used to deliver a medicinal product contained within. Thus, a PFS is a combination product – device and drug3. One additional feature of PFS systems is that they can be further incorporated into delivery devices of increasing complexity, such as autoinjectors, which are similarly expanding in popularity. This final combination product in a device body is perhaps one of the most challenging product-package systems for which to validate a CCI method per <1207.1>.
CCI Technologies and their Applications
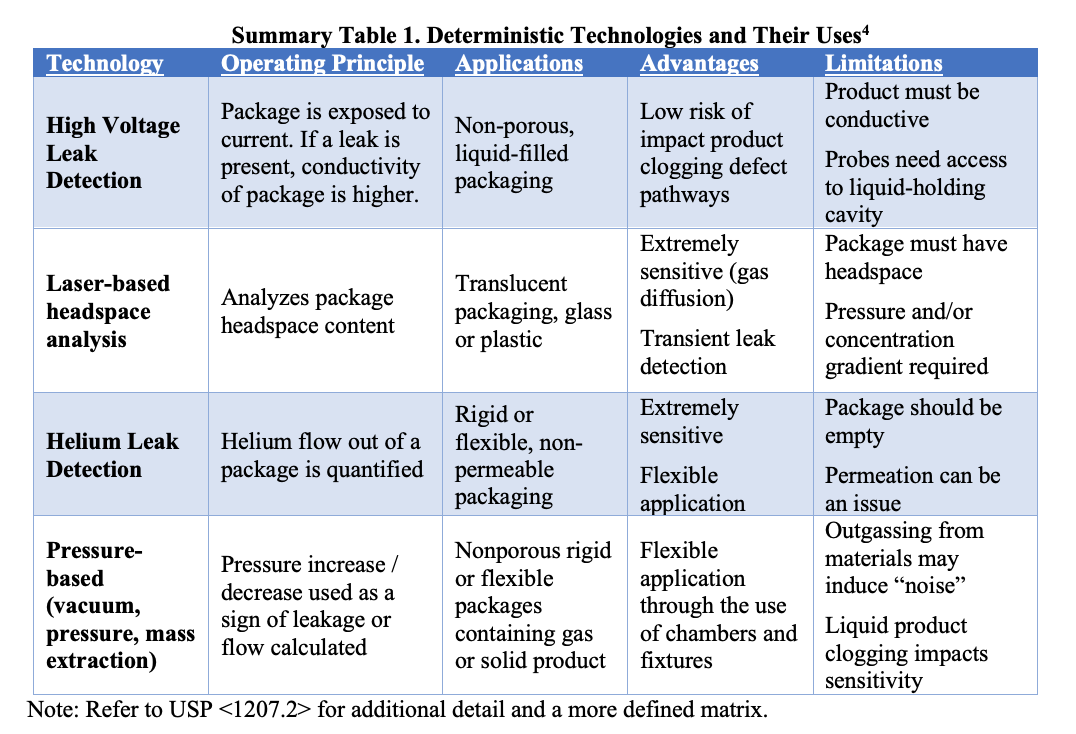
Understanding the challenges associated with testing a final autoinjection system requires some understanding of different CCI technologies, their applications, limitations, and sensitivities. All elements impacting method selection are not intended to be covered here, but it should be noted as a general rule that no one leak test technology is intended for all use cases. USP <1207.1> provides additional context on method selection and validation, and USP <1207.2> provides more detail on specific leak test methods. Each technology has advantages and limitations, and choosing the correct method is a function of what is feasible given the product, the package, and the unique study goals being considered at the time. A simplified matrix of deterministic leak test technologies and their general uses are summarized below.
The Silver Bullet Trap
The challenge in applying these deterministic leak test methods to a fully assembled autoinjector is most apparent in situations where CCI is being considered very late in the lifecycle: something to be performed on the final product-package system once other variables are finalized. This mentality likely stems from historical precedent. After all, isn’t a package just intended to hold the precious drug which had just been developed over the course of years? Even within the field of container testing, the regularity and scientific rigor with which integrity testing was pursued paled in comparison to other container tests, such as chemical characterization and permeation, which could lead to batch or lot-wide stability issues if left unchecked. Leakage, more anomalous in nature, could be considered more likely to impact a subset of a batch or lot.
Thus, the classic “study goal” for CCI testing was to demonstrate sterility for filing purposes. The 1999 packaging guidance released by FDA mentions container integrity as a requirement, but only directly ties it to the concept of microbiological integrity and sterility, hence the resulting popularity of microbial ingress as a test method. Often times not even validated with positive controls, routine dye or microbial ingress, became the norm. As a method, it did all a company could need for CCI.
However, as guidance and regulation surrounding CCI has evolved to include the concept of maximum allowable leakage limits (MALL) and more robust method development and validation practices, new technologies, deterministic in nature, have largely replaced the probabilistic dye and microbial ingress. The pitfall arises when under this new framework, an organization is seeking to develop and validate a modern test method that can cover all of the CCI concerns of the package and demonstrate integrity down to the MALL: a silver bullet. Due to the complexity of autoinjection devices and their contained doses, this is a daunting expectation.
The Challenge of Testing a Filled Autoinjector
The challenges of CCI testing autoinjection systems become apparent when considering the limitations introduced by the package system itself in tandem with the limitations inherent to the test technologies described above.
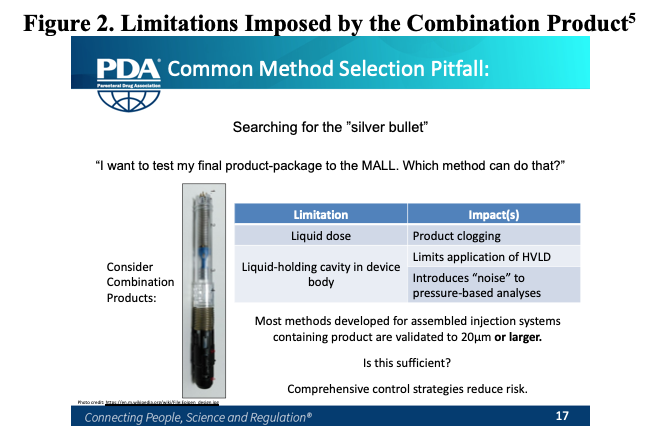
The liquid dose contained rules out helium leak detection as a feasible method. It also creates an issue with product clogging defect pathways. Although small molecule products, even saline solution, cause clogs, it is especially relevant for biologic products typically contained within these systems, which can be known to even clog needles6. This becomes a particular challenge during CCI method validation, where positive controls are used to demonstrate the ability of a method to find leaks of various sizes.
While high voltage leak detection (HVLD) is largely able to overcome the phenomenon of product clogging, as its method of detection is not reliant mass flowing through a defect, the device body surrounding the liquid holding PFS prevents immediate access. Thus, instrument probes are unable to scan the liquid holding container, and therefore, HVLD is not feasible for the final device. Similarly, the lack of headspace and laser access to the liquid-holding cavity prevents use of headspace analysis (HSA). This leads to the conclusion that a pressure-based method, such as vacuum decay, is the only method which may in fact be feasible.
Unfortunately, the issue with autoinjectors and pressure-based analysis does not end with the liquid dose causing clogging. Autoinjectors, with their large amount of dead-space inside and porous, plastic components, are extremely susceptible to generating noise in measurement due to outgassing. These combined limitations make method sensitivity a real concern. Based on experience, it is rare that a pressure-based method for a filled autoinjection system is even validated to 20μm. Often times, laser drilled holes of even 100μm or larger can be clogged, especially if time elapses between filling and testing.
Thus, the challenges presented by an autoinjection system leave no viable option for CCI testing to the MALL, which for the purposes of ensuring no microbial ingress, is typically considered to be around 0.2μm or a leak rate of about about 6.0E-6 mbar.l/s7,8.
There is no silver bullet.
CCI Control Strategies and Risk Mitigation
The CCI of any product-package system is going to be a function of its materials, design, and the processes that govern its creation, distribution, and use. For this reason, a modern consideration of CCI reaches much further back than the final product, encompassing every stage in a product’s lifecycle. This guidance is explicitly mentioned in USP <1207.1>, which establishes that CCI testing should be performed in package development and validation, product manufacturing, as well as stability and distribution. A comprehensive control strategy will identify potential risks to CCI as part of each of these lifecycle stages, and introduce controls to mitigate those risks.
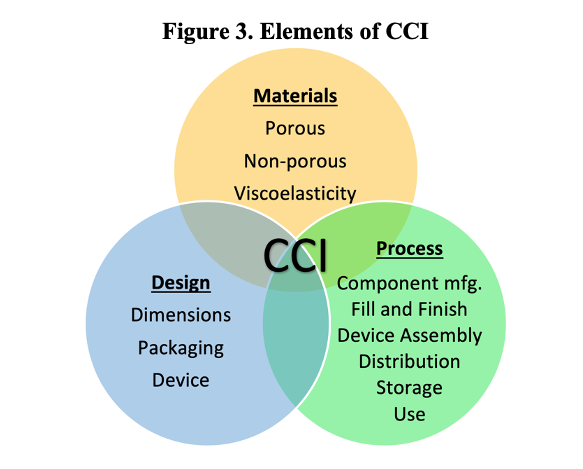
Not every part of a CCI control strategy needs to depend on leak testing. In fact, doing so could be considered “testing-in” quality. The preference is “to build-in” quality. For example, dimensional testing of incoming components may be incorporated as a control on the component manufacturing process. Similarly, visual inspection may remove grossly deformed packages or components, etc. There is a myriad of ways in which comprehensive control strategies can be built out, and additional information can be found in USP <1207.3> Seal Quality Tests, where additional non-leak tests are explored. However, the purpose of this article is specifically to discuss CCI testing strategies, which are critical to broader control strategies and lead to informed decision-making. In line with current USP guidance, the question may be asked: how can different technologies be implemented at different lifecycle stages to reduce risk of leakage?
This idea is referred to as development of the package integrity profile. A package integrity profile is an ongoing database of leak and seal quality tests moving through each of the main lifecycles. A well-established package integrity profile serves to demonstrate that the product-package is robust, in that it will maintain integrity across the variables experienced throughout the lifecycle. A case example of how three unique technologies can be used in support of developing an autoinjector’s package integrity profile is provided below.
Developing the Profile: Step #1 – Helium Leak Detection or Headspace Analysis
USP <1207.1> Lifecycle Stages: package development
Goal: demonstrate inherent integrity
Developing a package integrity profile starts before there is even a final package to speak of. Confirming primary packaging components are compatible with user requirements is key, and one such requirement is demonstration that the package can conform to the MALL. The concept of evaluating packages and packaging components to the MALL in the development phase is called establishing inherent integrity. As higher sensitivity methods are enabled by the use of empty packaging, this is often done.
In the case of an autoinjector, evaluation of the multiple sealing regions of the primary package would be of concern, as these would be considered the sterile barrier(s). In a PFS, there are numerous sealing regions, such as the plunger-barrel interface, staked needle to barrel bond, needle shield-barrel interface, and of course the needle to needle shield seal. Helium leak detection, with its sensitivity down to leak rates of E-11 mbar.l/s, and flexibility to test empty samples in unique ways, each of these seals can be isolated and tested.
Helium leak testing in the package development phase can help choose component suppliers, and establish dimensional specifications on plunger width for example, a critical dimension for sealing against the barrel. Studies evaluating individual rib performance can also be performed, a response to the increasingly common regulatory question regarding sterility during air transit of PFS9. This is even more relevant in the case of autoinjection systems, as the final device is likely to be assembled elsewhere from where the syringe was filled.
Headspace analysis can be equally sensitive, measuring gas concentration in package headspace over time. In cases where the intended product may be oxygen sensitive, for example, headspace analysis may be more reasonably applied to directly measure the ingress of oxygen into the system over time and under different variables.
By the completion of package development, there should be reasonable confidence that the primary package system, as it is intended to be used with respect to sourcing, assembly, storage, and distribution, will be suitable for use from an integrity standpoint. However, since helium is only suitable for testing on empty packages, its use is limited. Filled syringes cannot be tested, and as such, process variables downstream are unable to be evaluated directly by helium. Headspace analysis may run into similar challenges with filled syringes, whereby product precludes flow of gases through a defect site, though each case must be evaluated individually. Thus, moving into the lifecycle stage of manufacturing, a different method must be employed.
Developing the Profile: Step #2 – High Voltage Leak Detection
USP <1207.1> Lifecycle Stages: manufacturing, in-process, stability (PFS only)
Goal: incorporate manufacturing variables
Moving into manufacturing, and testing product-filled syringes, high voltage leak detection is widely used. By developing and validating a method on the standalone PFS, a new level of information is gathered and built into the package integrity profile. Variables experienced during the true manufacturing process (or trial runs, engineering batches, etc) can be evaluated, amongst other things.
From a sensitivity standpoint, methods developed for prefilled syringes using HVLD are typically validated to a limit of detection as low as 3μm in nominal orifice diameter. While this limit of detection may be substantially less sensitive than helium, the benefits provided by HVLD to the overall package integrity profile far outweigh this downside. This is part of the overall risk-mitigation approach. By demonstrating inherent integrity in package development, there should be reasonable confidence that the components, when assembled properly, do not leak. From a risk-assessment standpoint, then, risks such as cracking during plunger insertion or shipment, or fibers caught in the sealing region leading to leakage may be of concern. These also may be mitigated by non-testing control strategies, such as visual inspection, however, visual inspection does not replace the need for CCI. Both can be part of comprehensive control strategies, which should be evaluated on a case-by-case basis.
With the application of HVLD, samples off the manufacturing line is enabled, enabling the use of an in-process control test. There is also availability of 100% online applications in some cases. Additionally, manufactured PFS samples can be subjected to CCI testing before and after distribution simulation. If the PFS is a finished product in and of itself (some manufacturers offer both autoinjection and PFS configurations), the HVLD method could be enabled for CCI in lieu of sterility testing on stability.10

The limitation of this method, as previously discussed, is that probes need direct access to the liquid holding container. In this case, the PFS. Thus, when considering testing the PFS once assembled into the autoinjection device, HVLD is no longer applicable, which leads us to the third method which can be employed to contribute to the package integrity profile of the final product-package system.
Developing the Profile: Step #3 – Pressure-Based Analysis (Vacuum Decay)
USP <1207.1> Lifecycle Stages: package development, manufacturing, stability (Autoinjector only)
Goal: incorporate manufacturing variables
The complications of applying a pressure-based analysis such as vacuum decay on an autoinjector have already been discussed. Previously, though, it was in the context of seeking the “silver bullet”. In this case, the vacuum decay method on the final autoinjection system may indeed have relatively poor sensitivity when compared to leak sizes of concern. However, application of the method is being supported by the complete body of data collected through the development of the package integrity profile. Studies and learnings up to the point of final assembly should have substantially reduced risk of CCI failures present in PFS prior to assembly.
A validated vacuum decay method may be used for final device stability testing, given the appropriate risk-based justification. Sometimes, methods are developed on the final assembly, but filled with a water or placebo solution with similar properties. These surrogates have substantially less propensity to clog defects, and thus, methods are able to achieve a much lower limit of detection. Surrogate-filled systems allow for some important studies to be performed, such as validation of the device assembly process, ensuring risks during that process, such as syringe cracking due to high compressive forces, or needle-shield back-off. Similarly, propensity for defects such as cracking and cap back-off can be evaluated prior to and post distribution simulation to design or refine secondary and tertiary pack-out options for final autoinjector assemblies. Lastly, incorporation of water-filled samples into a broader stability testing matrix including drug product-filled PFS, may be of benefit, as it can demonstrate “functional stability” of the autoinjection unit over time.
Concluding Statements
Summary Table 2 below summarizes how three technologies can work in tandem to support the same final product, as well as some studies that can be performed using each of the 3 technologies.
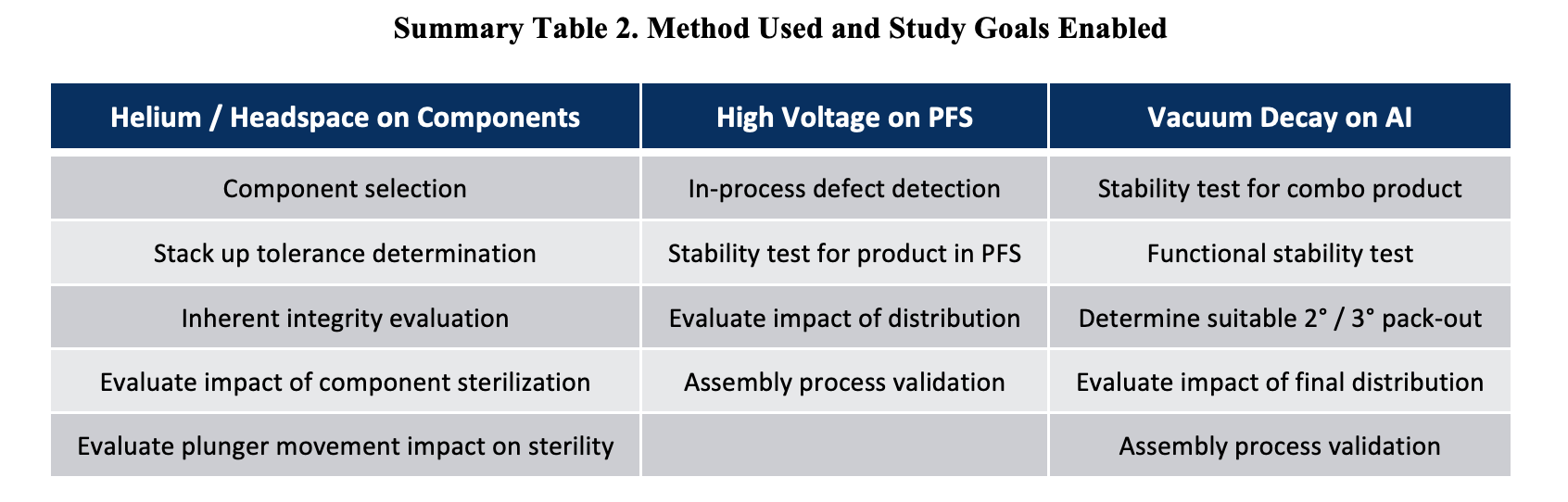
Although risk assessment and control strategies will vary widely according to the product-package or organization, and CCI control should be planned on a case-by-case basis, when it comes to assessing autoinjectors for CCI, universal challenges can be expected. By applying modern expectations of container closure integrity testing according to current guidance, such as USP <1207>, it becomes clear that a single method is unlikely able to support all critical concerns of the package system (MALL). Whereas previously CCI was frequently performed on the final system using a single method, a lifecycle approach to CCI provides a framework for better risk reduction and demonstration that a package is suitable for its intended use. In much the way that an analytical lab has multiple instruments to evaluate the stability of a drug product, an ideally suited package testing lab has multiple technologies as well. Multiple methods, employed creatively, can generate a package integrity profile used; an ongoing database of leak and seal quality tests used to finalize, improve, monitor, and ultimately prove integrity across lifecycle stages.
Reference Documents
- Ugalmugle, S. (2019, July). Prefilled Syringes Market Statistics: 2019-2025 Global Forecasts Report. Retrieved from https://www.gminsights.com/industry-analysis/prefilled-syringes-market
- Dorgan, C. (2018, June). Designing Container Closure Systems for Enhanced Functionality and Usability. Presentation at the PDA Container Closure Conference, Bethesda, MD.
- Frequently Asked Questions About Combination Products. (2020, April 9). Retrieved April 17, 2020, from https://www.fda.gov/combination-products/about-combination-products/frequently-asked-questions-about-combination-products#CP
- S. Pharmacopoeia. USP 41 <1207.2>. Package Integrity Leak Test Technologies. United States Pharmacopoeial Convention, Inc.: Rockville, MD, 2018
- Zurawlow, B. (2020, February). Deck 8 – Test Method Selection and Application. Presentation at the PDA Container Closure Integrity Basic Course, following the Parenteral Packaging Conference, Gothenburg, Sweden.
- De Bardi, M., Müller, R., Grünzweig, C., Mannes, D., Boillat, P., Rigollet, M., … & Yang, K. (2018). On the needle clogging of staked-in-needle pre-filled syringes: Mechanism of liquid entering the needle and solidification process. European Journal of Pharmaceutics and Biopharmaceutics, 128, 272-281.
- Kirsch, L. E., Nguyen, L., Moeckly, C. S., & Gerth, R. (1997). Pharmaceutical container/closure integrity II: The relationship between microbial ingress and helium leak rates in rubber-stoppered glass vials. PDA Journal of Pharmaceutical Science and Technology, 51(5), 195-202.
- S. Pharmacopoeia. USP 41 <1207.1>. Package Integrity Testing in The Product Life Cycle—Test Method Selection and Validation. United States Pharmacopoeial Convention, Inc.: Rockville, MD, 2018
- Candau-Chacon, R.(2018, October). Designing Container Closure Systems for Enhanced Functionality and Usability. Presentation at the PDA Annual Conference on Pharmaceutical Microbiology, Bethesda, MD.
- Container and Closure System Integrity Testing in Lieu of Sterility Testing as a Component of the Stability Protocol for Sterile Products, Container and Closure System Integrity Testing in Lieu of Sterility Testing as a Component of the Stability Protocol for Sterile Products (2008). Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/container-and-closure-system-integrity-testing-lieu-sterility-testing-component-stability-protocol

