Written By BRIAN MULHALL / Chief Executive Officer
As we all know, the USP has made multiple revisions to virtually all parts of the package-system qualification testing procedures over the past 5-7 years. USP Spectral Transmission Testing (often referred to as Light Percent Testing) has not been spared and like many of the container specific USP procedures, it is a bit more complicated than it used to be. Understanding how the USP Spectral Transmission testing is applicable to a product package system and the key steps in the testing process will help to ensure that the regulatory requirements are being met.
As referenced in USP <659> Packaging and Storage Requirements, a light-resistant container “protects the contents from the effects of light by virtue of the specific properties of the material of which it is composed”. How light resistance is achieved can vary depending on the system. For plastic bottles, light resistance is typically based upon the infusion of titanium dioxide into the resin such that the container is colored white. Think of all the white HDPE containers on the shelf of the local drug store. For glass containers, amber colorant is the typically method. The basic interpretation of the regulatory guidelines are relatively straightforward: if your product is light sensitive and the container or package system is designed to prevent light intrusion, then the container or package system is subject to USP <671> Spectral Transmission Testing requirements. Various references to the testing guidelines can be found in USP <671>, USP <659>, USP <660>, and USP <661.2>. Specific USP test procedure steps are as follows:
Apparatus (instrument for testing): Spectral Transmission Testing is done using a UV-Visible Spectrophotometer capable of analyzing samples from 290-450 nanometers. For certain sample types (ex. Plastic container), the instrument should be fitted with an integrating sphere sample holder that enables the mounting of a sample section.
Key Procedural Steps: Typically samples being tested are plastic or glass containers, The sample preparation steps vary slightly based upon the sample type. For plastic containers, cut a circular section of the container from an area of the bottle that represents the average thickness of the container. Typically the sample section is taken from the sidewall area. While there is no size directive, it is suggested that it be in the range of 2-3 inches in diameter with the key being that it must be large enough to be mounted on the sample holder of the instrument ensuring that the instrument slit is completely covered. Prior to placement in the UV-Visible Spectrophotometer, the sample should be washed and dried with care taken to not scratch the surface area. Specific USP directions for sample mounting are stated as follows: “Place the section in the spectrophotometer with its cylindrical axis parallel to the plane of the slit and approximately centered with respect to the slit. When properly placed, the light beam is normal to the surface of the section and reflection losses are at a minimum.”
With today’s instruments that operate using OEM provided 21 CFR Part 11 Software, in most cases you will have the ability to have the specific test method for the sample type programmed into the instruments. The specific test parameters state that the light percent shall be measured from 290-450 nm with a recording interval of not less than 20 nm. After completion of the sample test scan, the results will be compared to the USP assigned acceptance criteria for the sample type to determine a meets or does not meet the assigned specification result.
Acceptance Criteria: Acceptance criteria are assigned based upon the sample type, container size and the intended use (product type) of the container.
Plastic Container, Regardless of Size, intended for an Oral or Topical Dosage Form: The observed spectral transmission does not exceed 10% at any wavelength in the range of 290–450 nm.
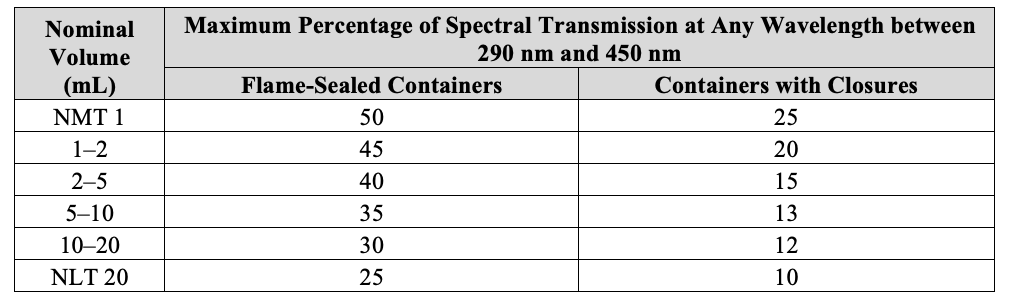
Plastic Container intended for Parenteral Dosage Form: The acceptance criteria will be based upon the nominal size of the container as outlined in the following table:
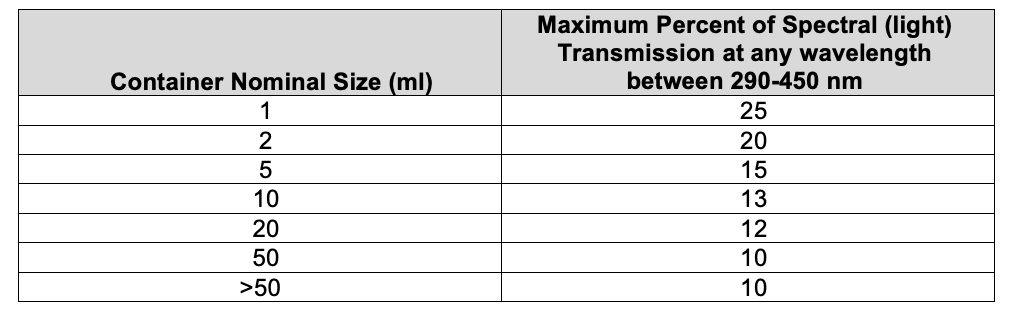
Glass Containers for NON – Parenteral Products: The observed spectral transmission does not exceed 10% at any wavelength in the range of 290–450 nm, irrespective of the type and capacity of the glass container.
Glass Containers for Parenteral Products The observed spectral transmission does not exceed the limits outlined in the following table noting that nominal volume of the container being a key component.