Package Development and Validation Studies
USP <1207> discusses best practices for incorporating CCI throughout the lifecycle of a productpackage system, starting with package development and moving through distribution and shelflife stability studies. In a previous blog, the benefits of studies early in the lifecycle, specifically package development and assembly process validation, were highlighted.
One primary example of container closure integrity testing implemented in this phase is a capping optimization or validation study. In these programs, vials are capped either according to final capping parameters, or in groups at a range of parameters if they have yet to be finalized. By evaluating these samples by a very sensitive container closure integrity test, such as headspace analysis or helium leak detection, confidence in the ability of the system to maintain an integral seal can be gained.
Oftentimes, this type of test program is accompanied by residual seal force testing, which indirectly measures the force the stoppers is applying to the top of the vial, as well as calculations of % compression for the elastomer. Ultimately, residual seal force, % compression, and to a large extent container closure integrity have to do with the dimensional, physical fit between the vial and the elastomeric closure, compressed and held in place by the aluminum crimp seal. Understanding and characterizing the variables that can impact this relationship is critical in the comprehensive characterization of container closure integrity of a product-package system.
Sources and Impacts of Leakage
It is generally accepted that as leakage is a continuum, to some degree, all packages leak. However, the sources and therefore opportunities to prevent and limit leakage are myriad. Typically, leakage originates from one of two areas defects, such as a crack in vial, deformed stopper, etc., and the seal area, which is influenced by a range of factors.
Temperature, processing variables such as sterilization, crimping parameters, and cleanliness can all have substantial impact on the integrity of a vial. The importance and impact of these variables, however, can pale in comparison to the relevance of the dimensional fit between the sealing components – the compression of the elastomer against the glass.
Vial Seal Geometry and Critical Dimensions
The “seal” of a vial is actually a combination of multiple sealing areas, together, or sometimes independently, creating the package barrier. The valve seal is formed between the plug or legs of a stopper and the inner neck of the vial. The land seal is formed between the flange of the stopper and flat top of the vial mouth. The transition seal is formed at the junction between the two, sometimes aided by vial blowback designs. The image below highlights each relevant vial seal.
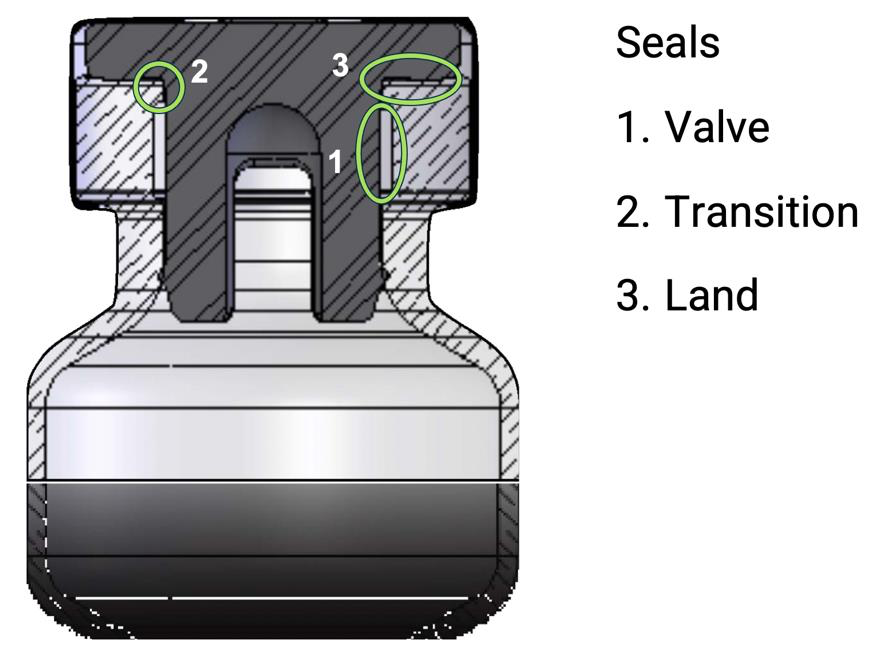
Valve Seal Dimensions
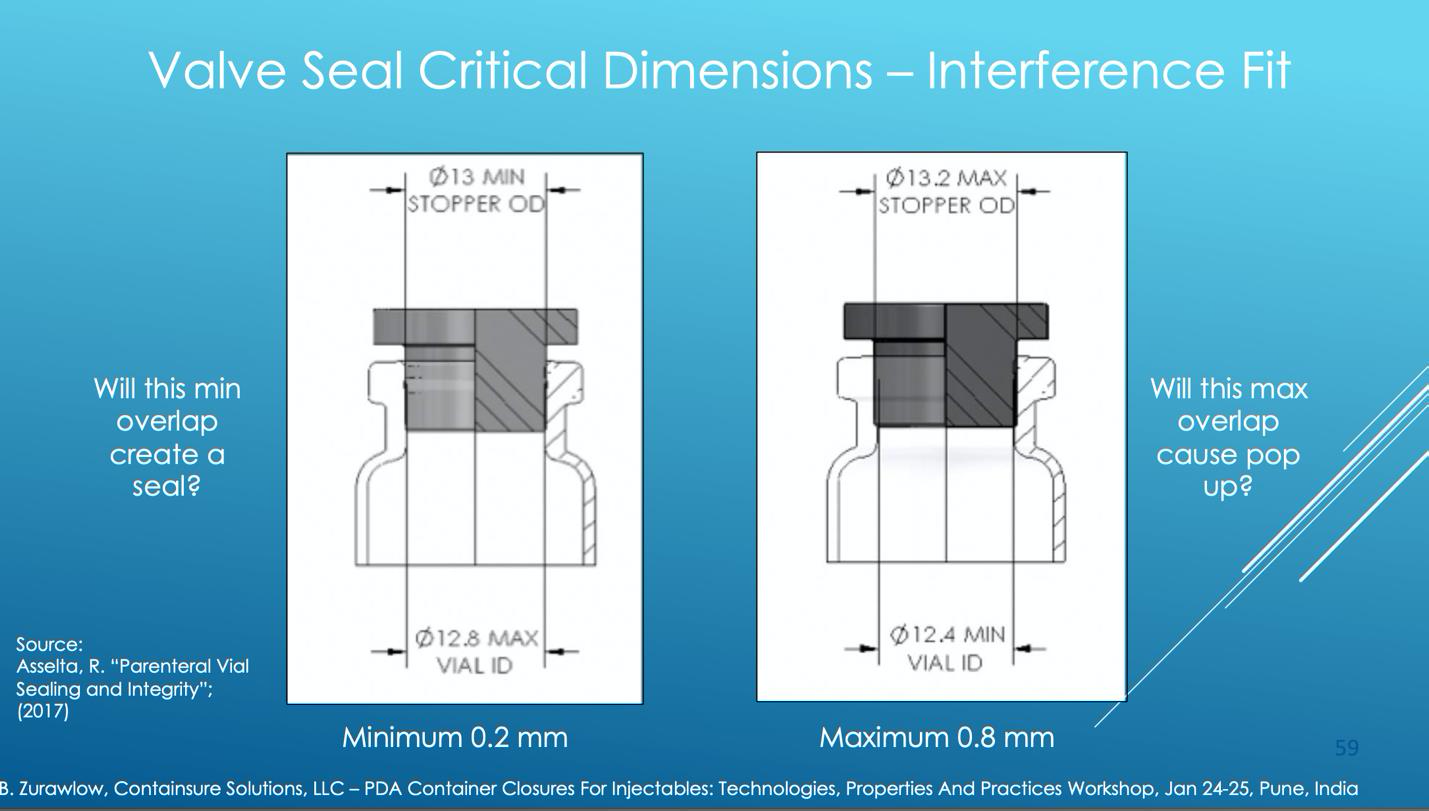
The valve seal is important for positioning the stopper into the vial, and creating a temporary seal prior to final capping, something especially relevant for lyo applications. The efficacy of this seal is a function of the dimensional overlap, or interference fit, between the stopper flange and the vial neck inner diameter. As this seal is not reliant on application of the crimp seal, and thus additional mechanical compression, the dimensional fit of these components almost entirely dictates the quality of the seal.
The figure below highlights the impact of vial neck inner diameter and stopper plug outer diameter. If the overlap is too low, (widest vial neck with smallest stopper) a good seal may not be formed. If the overlap is too high, (smallest vial neck, widest stopper) the stopper may not fit at or “stopper pop-up” may occur, where the stopper pops up or out before the crimp is applied.
Land Seal Dimensions
The land seal of a vial is typically considered the primary final seal for maintenance of container closure integrity. Like the valve seal, it is largely impacted by dimensional overlap. However, unlike the valve seal, the land seal is formed by application of mechanical compression during the crimping process. In other words, the land seal is also a result of a process, which is measurable and therefore controllable. Understanding the process through a capping study is key, but understanding and controlling component dimensions can be just as critical.
In typical capping systems, the stopper is compressed against the mouth of the vial, and held in a compressed state by an aluminum crimp seal. The amount of compression that occurs, and thus the seal, is impacted by the dimensional “stack-up” of these components, as illustrated below.
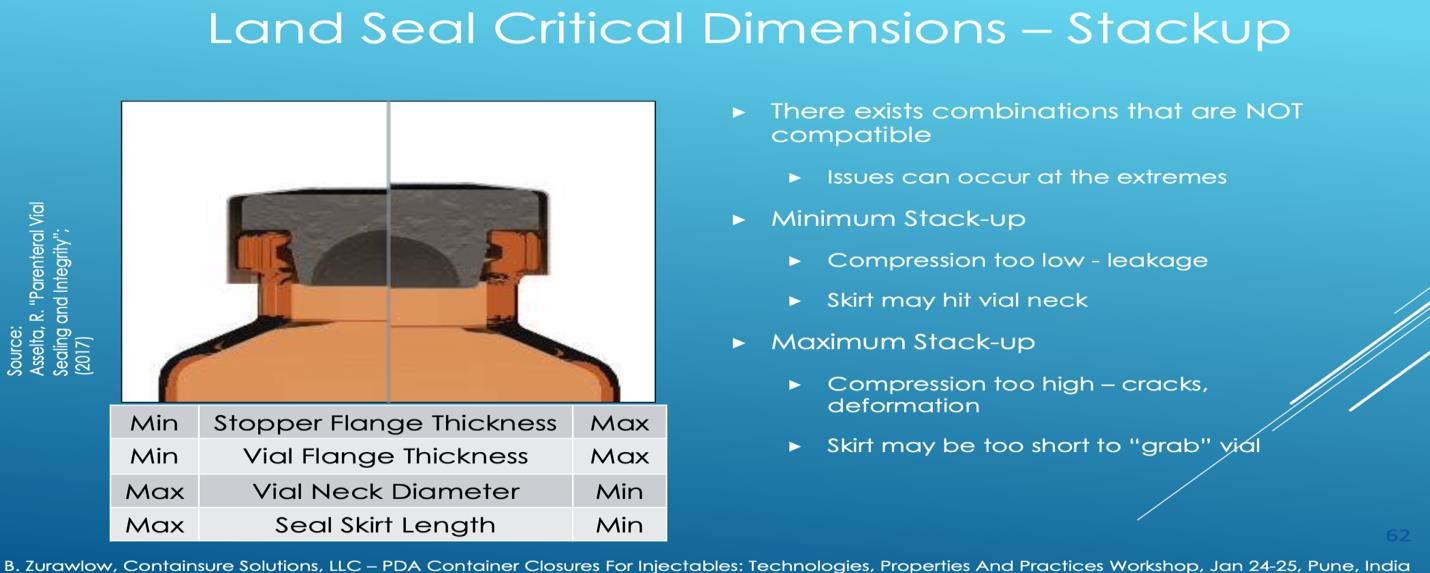
Dimensional variation can lead to variation in seal quality and potentially issues. In instances where the thinnest stopper flange and vial flange are compressed together using the longest aluminum skirt, poor compression can be obtained and issues with the excess aluminum scoring or cracking the vial neck can occur as it is tucked under during the sealing process. On the other extreme, if the thickest stopper and vial flanges are crimped, very high compression can be obtained. Short crimp seal skirts may be too short to even wrap around the base of the vial neck.
Dimensional Variability
This variation in dimensional components can be wide amongst designs and suppliers of otherwise similarly sized or marketed components. It is important to select components that complement each other to ensure that during the random process of manufacturing, a substantial portion of the batch is not compromised due to dimensional incompatibility. This variation is also inherent between and within lots of components, which is why critical dimensional specifications and tolerances are established for pharmaceutical packaging components.
Dimensional Variability in CCI Studies and Control Strategies
Assessing component variability and incorporating it into a CCI study can help elucidate and mitigate risk of dimension-related seal-ability issues. As a straightforward example, lots of vials, stoppers, and seals can be measured for critical dimensions using calipers, optical comparators, or complex digital measurement systems such as the Keyence maintained at CS Analytical. Dimensional extremes can be paired and the resulting packages tested for compression, RSF, and leakage to establish confidence that across the anticipated dimensional tolerances, package components will create an adequate seal.
While a point-in-time study is immensely valuable, USP <1207> also discusses the importance of comprehensive CCI control strategies, not all of which is built upon directly testing container closure integrity, or leakage. RSF as an in-process check during manufacturing is a good example. Another common control strategy is incoming inspection of components, a portion of which typically includes analysis of component critical dimensions. By ensuring through a comprehensive study that across the anticipated tolerance range CCI can be assured. By following up with continued verification that received components adhere to those dimensional specifications, one can begin to see how CCI control strategies take shape and provide regulatory value.
The team at CS Analytical has years of experience pioneering and implementing comprehensive CCI programs for clients with products that span lifecycle and regulatory phases. With increasing awareness of industry and regulators, the frequency and scientific value of these programs is expected to increase for the forseeable future. In future blogs, other variables related to seal integrity, such as temperature, will be explored in similar context to equip readers with a more comprehensive understanding of CCI as a concept and more than just a “test”.

