About admin
This author has not yet filled in any details.So far admin has created 118 blog entries.
Quality Data Reviewer – FDA Regulated, cGMP Facility – Full-Time and Part-time Opportunities Available (On-Site)
Full-Time and Part-time Opportunities Available for Quality Data Review (On-Site)
CS Analytical Laboratory Announces Expansion to their Package Distribution Testing Service Fully Operational
CLIFTON, N.J., May 20, 2025 /PRNewswire/ -- CS Analytical Laboratory, the
Business Ops Associate
Company Information: Located in Clifton, New Jersey, CS Analytical is
Understanding the New European Pharmacopoeia 2.4.35 Chapter for Elemental Impurities in Plastic Materials
Author: Ronak Patel, Analytical Chemistry Manager Elemental impurities in
Magna Mike Thickness Guage Testing
By Meaghan Mancini / Laboratory Analyst II The Magna
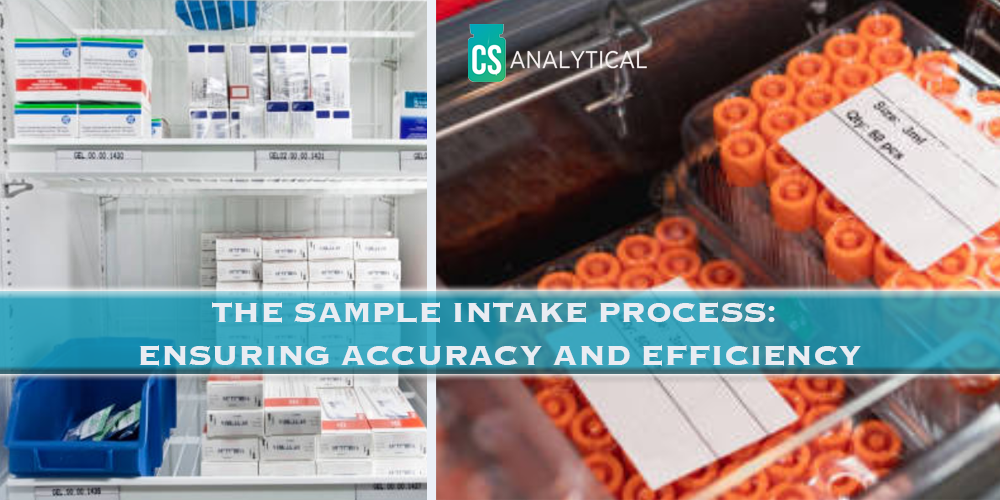
The Sample Intake Process: Ensuring Accuracy and Efficiency
Written By Jacqueline Zsoldos / Quality Administrator When samples
CS Analytical Signs IT System Support Agreement with E-2 Consulting
CLIFTON, N.J., April 8, 2025 /PRNewswire/ -- CS Analytical Laboratory,
CS Analytical Extends High Voltage Leak Testing Capabilities with Addition of Nikka Densok HDL-1 HVLD Test System
CLIFTON, N.J., March 25, 2025 /PRNewswire/ -- CS Analytical
Understanding Cleaning Validation in Pharmaceutical Manufacturing
Written By Ronak Patel / Manager, Analytical Chemistry Cleaning