About admin
This author has not yet filled in any details.So far admin has created 124 blog entries.
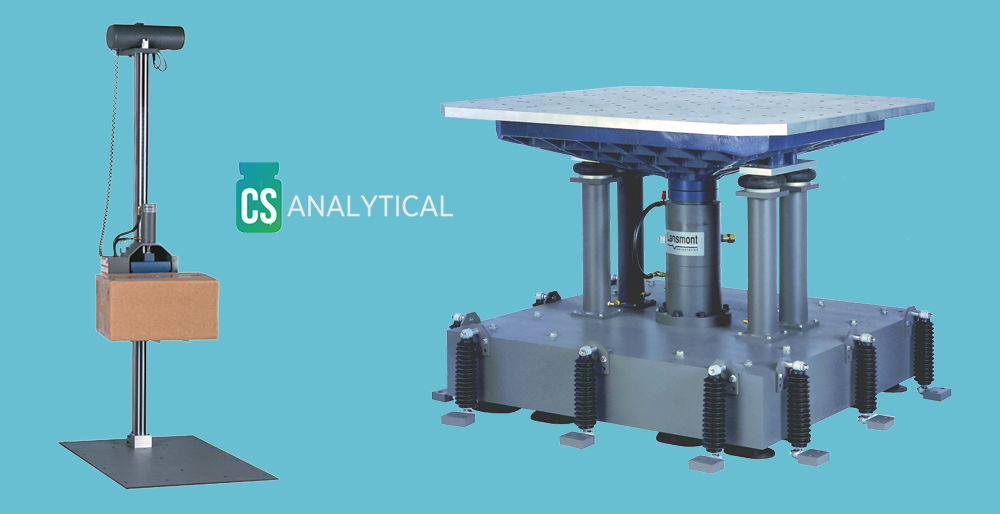
Package Distribution Testing Test Method Selection: ASTM 4169 or ISTA Standard
Package (shipment) Distribution Testing is designed to simulate the distribution environment
CS Analytical Expands the Quality Team with Recent Hire
CLIFTON, N.J., Oct. 9, 2023 /PRNewswire-PRWeb/ CS Analytical Laboratory,
CS Analytical Expands USP 1207 Container Closure Integrity Testing Programs for IV Bag Systems
CLIFTON, N.J., Sept. 19, 2023 /PRNewswire-PRWeb/ -- CS Analytical
CS Analytical Partners with Instron for Webinar on Functional and Mechanical Testing of Drug Delivery Devices
CLIFTON, N.J., Sept. 6, 2023 /PRNewswire-PRWeb/ -- CS Analytical Laboratory,
Differentiating USP <671> Permeation Test Methods – Part One
Understanding the permeation testing requirements of the USP <671> Container
Critical Reasons for the early adoption of USP <661.1> and USP <661.2>
Plastics are a wide range of synthetic or semi-synthetic materials
USP 661.1 and USP 661.2 | Frequently Asked Questions
Are the 661.1 and 661.2 Chapters now effective and required?
CS Analytical Laboratory Announces Notable Achievements for 2023
CLIFTON, N.J. (PRWEB) JUNE 28, 2023 CS Analytical Laboratory, the world’s
CS Analytical Laboratory Continues Growth & Expansion with New Hires
CLIFTON, N.J. (PRWEB)June 14, 2023 CS Analytical Laboratory, the world’s