CS Analytical – How We Are Different
Even as CS Analytical is truly unique as the world’s
Extensive Knowledge & Experience with a Wide Variety of Drug Product Package System
The CS Analytical is the only FDA Regulated, cGMP contract
Meet members of our All-Star team of package professionals
The CS Analytical is the only FDA Regulated, cGMP
Dose Accuracy Testing
Understanding Deliverable Volume and Uniformity of Dosage Deliverable volume
Critical Considerations in Choosing a USP <1207> Container Closure Integrity Testing Laboratory
Written By BRIAN MULHALL / Chief Executive Officer As outlined
USP Chapters Specific to Container & Package System Testing
In review of the current and pending requirements for USP
Common Seal Strength Testing Methods
Seal-strength testing can play a critical role when working to
A Summary of USP <671> Permeation Test Methods
Understanding the permeation testing requirements of the USP <671>
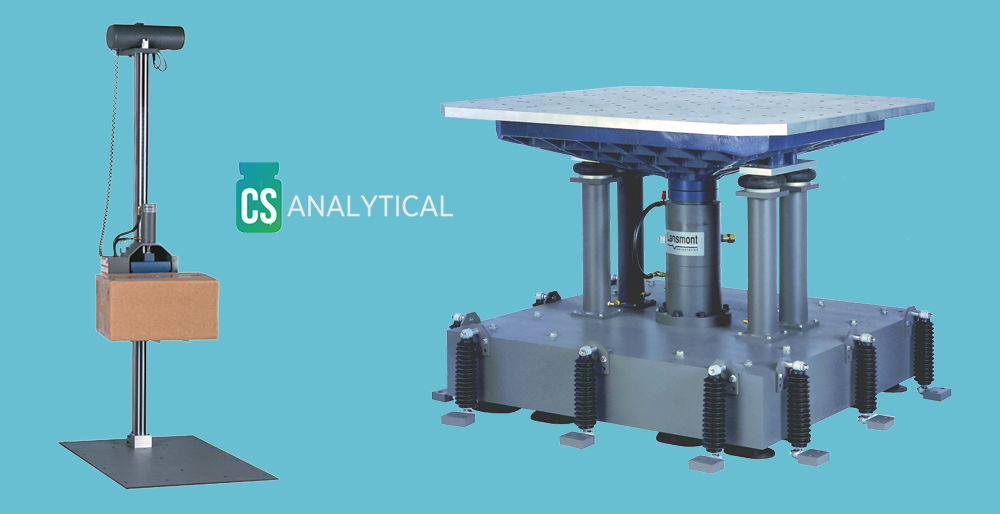
Package Distribution Testing Test Method Selection: ASTM 4169 or ISTA Standard
Package (shipment) Distribution Testing is designed to simulate the distribution environment