About admin
This author has not yet filled in any details.So far admin has created 125 blog entries.
Understanding the New European Pharmacopoeia 2.4.35 Chapter for Elemental Impurities in Plastic Materials
Author: Ronak Patel, Analytical Chemistry Manager Elemental impurities in
Magna Mike Thickness Guage Testing
By Meaghan Mancini / Laboratory Analyst II The Magna
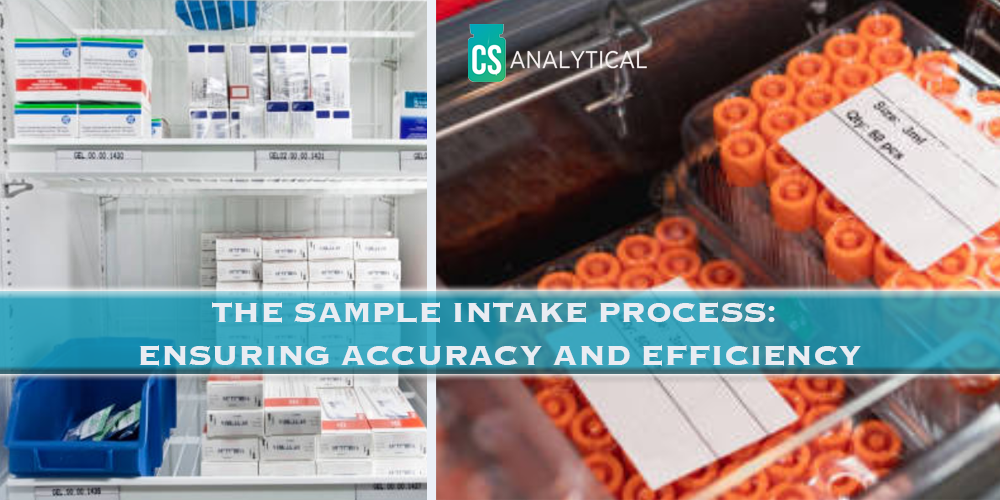
The Sample Intake Process: Ensuring Accuracy and Efficiency
Written By Jacqueline Zsoldos / Quality Administrator When samples
CS Analytical Signs IT System Support Agreement with E-2 Consulting
CLIFTON, N.J., April 8, 2025 /PRNewswire/ -- CS Analytical Laboratory,
CS Analytical Extends High Voltage Leak Testing Capabilities with Addition of Nikka Densok HDL-1 HVLD Test System
CLIFTON, N.J., March 25, 2025 /PRNewswire/ -- CS Analytical
Understanding Cleaning Validation in Pharmaceutical Manufacturing
Written By Ronak Patel / Manager, Analytical Chemistry Cleaning
A history of the United States Pharmacopeia (USP)
Written By Rohan Kumar / Laboratory Analyst 1 Author
Ensure Your Plastic Materials of Construction and Package Systems are Qualified Under USP 661.1 and 661.2 Without Delay.
Written By Ronak Patel, Manager, Analytical Chemistry The clock
Understanding USP 660 Glass Testing – Autoclave Use and Test Timing
Written By FATIMA YACOUBI, Laboratory Analyst III / Project